Ethanol: Chemical compound
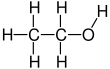
Ethanol, also known as ethyl alcohol, grain alcohol or just alcohol, is a flammable, colorless chemical compound.
Its chemical formula is C2H5OH, also written as C2H6O. It is the active part of alcoholic drinks, which are drunk in most cultures worldwide. It is also used as a solvent because it can dissolve many other chemicals and is not very toxic. Yeast makes most of the ethanol that people use.
| |||
| |||
| Names | |||
|---|---|---|---|
| Pronunciation | /ˈɛθənɒl/ | ||
| Systematic IUPAC name ethanol | |||
| Other names Absolute alcohol alcohol cologne spirit drinking alcohol ethylic alcohol EtOH ethyl alcohol ethyl hydrate ethyl hydroxide ethylol grain alcohol hydroxyethane methylcarbinol | |||
| Identifiers | |||
3D model (JSmol) | |||
| 3DMet | |||
| Beilstein Reference | 1718733 | ||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.000.526 | ||
| Gmelin Reference | 787 | ||
IUPHAR/BPS | |||
PubChem CID | |||
| UNII | |||
| UN number | UN 1170 | ||
CompTox Dashboard (EPA) | |||
SMILES
| |||
| Properties | |||
| C2H6O | |||
| Molar mass | 46.07 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Density | 0.7893 g/cm3 (at 20 °C) | ||
| Melting point | −114.14 ± 0.03 °C (−173.45 ± 0.05 °F; 159.01 ± 0.03 K) | ||
| Boiling point | 78.24 ± 0.09 °C (172.83 ± 0.16 °F; 351.39 ± 0.09 K) | ||
| miscible | |||
| log P | −0.18 | ||
| Vapor pressure | 5.95 kPa (at 20 °C) | ||
| Acidity (pKa) | 15.9 (H2O), 29.8 (DMSO) | ||
| −33.60·10−6 cm3/mol | |||
Refractive index (nD) | 1.3611 | ||
| Viscosity | 1.2 mPa·s (at 20 °C), 1.074 mPa·s (at 25 °C) | ||
| 1.69 D | |||
| Hazards | |||
| NFPA 704 | | ||
| Flash point | 14 °C (Absolute) | ||
| U.S. Permissible exposure limit (PEL) | TWA 1000 ppm (1900 mg/m3) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||

Ethanol fuel
Ethanol fuel can be used instead of gasoline in cars and other engines. Engines can use pure ethanol or ethanol mixed with gasoline.
In Brazil, ethanol fuel made from sugar cane provides 18 percent of the country's fuel for cars. Because of this, Brazil does not have to buy oil from other countries. To do this, Brazil cut down much of the rain forests to grow more sugar cane, which is then fermented into Ethanol.
Most cars in the U.S. can run on fuels that have of up to 10% ethanol in them. Car makers like Ford, DaimlerChrysler, and GM also make vehicles specifically designed to run on higher ethanol blends. Some of their engines can run on up to 85% ethanol (E85). By mid-2006, there were about six million E85-compatible vehicles on U.S. roads.
References
Other websites
- US E85 refueling map Archived 2007-09-21 at the Wayback Machine
- International Chemical Safety Card 0044
- Molview from bluerhinos.co.uk Archived 2008-09-25 at the Wayback Machine See Ethanol in 3D (may take over a minute to load using a dial-up connection)
This article uses material from the Wikipedia Simple English article Ethanol, which is released under the Creative Commons Attribution-ShareAlike 3.0 license ("CC BY-SA 3.0"); additional terms may apply (view authors). Content is available under CC BY-SA 4.0 unless otherwise noted. Images, videos and audio are available under their respective licenses.
®Wikipedia is a registered trademark of the Wiki Foundation, Inc. Wiki Simple English (DUHOCTRUNGQUOC.VN) is an independent company and has no affiliation with Wiki Foundation.