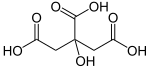
Citric Acid: Weak organic acid
Citric acid is a weak organic acid.
It can be found in citrus fruits. It is used by organisms for Krebs cycle. It acts like a preservative when added to food. It is also used to add a sour (acidic) taste to foods and soft drinks. In the European Union it is known as E 330, as a food additive.
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name 2-Hydroxypropane-1,2,3-tricarboxylic acid | |||
| Identifiers | |||
| |||
3D model (JSmol) | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.000.973 | ||
| EC Number |
| ||
| E number | E330 (antioxidants, ...) | ||
IUPHAR/BPS | |||
| KEGG | |||
PubChem CID | |||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA) | |||
SMILES
| |||
| Properties | |||
| C6H8O7 | |||
| Molar mass | 192.123 g/mol (anhydrous), 210.038 g/mol (monohydrate) | ||
| Appearance | Crystalline white solid | ||
| Odor | Odorless | ||
| Density | 1.665 g/cm3 (anhydrous) 1.542 g/cm3 (18 °C, monohydrate) | ||
| Melting point | 156 °C (313 °F; 429 K) | ||
| Boiling point | 310 °C (590 °F; 583 K) decomposes from 175 °C | ||
| 117.43 g/100 mL (10 °C) 147.76 g/100 mL (20 °C) 180.89 g/100 mL (30 °C) 220.19 g/100 mL (40 °C) 382.48 g/100 mL (80 °C) 547.79 g/100 mL (100 °C) | |||
| Solubility | soluble in acetone, alcohol, ether, ethyl acetate, DMSO insoluble in C 6H 6, CHCl3, CS2, toluene | ||
| Solubility in ethanol | 62 g/100 g (25 °C) | ||
| Solubility in amyl acetate | 4.41 g/100 g (25 °C) | ||
| Solubility in diethyl ether | 1.05 g/100 g (25 °C) | ||
| Solubility in 1,4-Dioxane | 35.9 g/100 g (25 °C) | ||
| log P | −1.64 | ||
| Acidity (pKa) | pKa1 = 3.13 pKa2 = 4.76 pKa3 = 6.39, 6.40 | ||
Refractive index (nD) | 1.493–1.509 (20 °C) 1.46 (150 °C) | ||
| Viscosity | 6.5 cP (50% aq. sol.) | ||
| Structure | |||
| Monoclinic | |||
| Thermochemistry | |||
| Std enthalpy of formation ΔfH | −1548.8 kJ/mol | ||
| Std enthalpy of combustion ΔcH | −1960.6 kJ/mol −1972.34 kJ/mol (monohydrate) | ||
| Standard molar entropy S | 252.1 J/(mol·K) | ||
| Specific heat capacity, C | 226.51 J/(mol·K) (26.85 °C) | ||
| Pharmacology | |||
ATC code | A09AB04 (WHO) | ||
| Hazards | |||
| Main hazards | skin and eye irritant | ||
| NFPA 704 | | ||
| Explosive limits | 8% | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||

Carl Wilhelm Scheele was the first who could extract citric acid from lemons, in 1782. The substance was probably known to alchemists, perhaps with a different name. The Arabian alchemist Geber is said to have discovered citric acid in the 9th century. Citric Acid contains 6 Carbon atoms, 8 Hydrogen atoms and 7 Oxygen atoms. Its chemical formula is C6H8O7.
Main uses
- As a water softener
- It is often used in detergents, to avoid the smell of acid, esp. Acetic acid
- As a preserving agent
- Citric acid and its salts prevent blood clotting. Blood donations are kept liquid using citric acid.
References
Other websites
This article uses material from the Wikipedia Simple English article Citric acid, which is released under the Creative Commons Attribution-ShareAlike 3.0 license ("CC BY-SA 3.0"); additional terms may apply (view authors). Content is available under CC BY-SA 4.0 unless otherwise noted. Images, videos and audio are available under their respective licenses.
®Wikipedia is a registered trademark of the Wiki Foundation, Inc. Wiki Simple English (DUHOCTRUNGQUOC.VN) is an independent company and has no affiliation with Wiki Foundation.