Zinc Chloride
Zinc chloride is the name of inorganic chemical compounds with the formula ZnCl2·nH2O, with n ranging from 0 to 4.5, forming hydrates.
Zinc chloride, anhydrous and its hydrates are colorless or white crystalline solids, and are highly soluble in water. Five hydrates of zinc chloride are known, as well as four forms of anhydrous zinc chloride. This salt is hygroscopic and even deliquescent. Zinc chloride finds wide application in textile processing, metallurgical fluxes, and chemical synthesis. No mineral with this chemical composition is known aside from the very rare mineral simonkolleite, Zn5(OH)8Cl2·H2O.
 Anhydrous | |
 Monohydrate | |
 | |
| Names | |
|---|---|
| IUPAC name Zinc chloride | |
Other names
| |
| Identifiers | |
| |
3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.028.720 |
| EC Number |
|
PubChem CID | |
| RTECS number |
|
| UNII | |
| UN number | 2331 |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| ZnCl2 | |
| Molar mass | 136.315 g/mol |
| Appearance | White hygroscopic and very deliquescent crystalline solid |
| Odor | odorless |
| Density | 2.907 g/cm3 |
| Melting point | 290 °C (554 °F; 563 K) |
| Boiling point | 732 °C (1,350 °F; 1,005 K) |
| 432.0 g/100 g (25 °C) 615 g/100 g (100 °C) | |
| Solubility | soluble in ethanol, glycerol and acetone |
| Solubility in ethanol | 430.0 g/100 ml |
| −65.0·10−6 cm3/mol | |
| Structure | |
| Tetrahedral, linear in the gas phase | |
| Pharmacology | |
| B05XA12 (WHO) | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards | Moderately toxic, irritant |
| GHS labelling: | |
   | |
| Danger | |
| H302, H314, H410 | |
| P273, P280, P301+P330+P331, P305+P351+P338, P308+P310 | |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
|
LC50 (median concentration) | 1260 mg/m3 (rat, 30 min) 1180 mg-min/m3 |
| NIOSH (US health exposure limits): | |
PEL (Permissible) | TWA 1 mg/m3 (fume) |
REL (Recommended) | TWA 1 mg/m3 ST 2 mg/m3 (fume) |
IDLH (Immediate danger) | 50 mg/m3 (fume) |
| Safety data sheet (SDS) | External MSDS |
| Related compounds | |
Other anions | |
Other cations | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Structure and properties
Four crystalline forms (polymorphs) of ZnCl2 are known: α, β, γ, and δ. Each case features tetrahedral Zn2+ centers.
| Form | Crystal system | Pearson symbol | Space group | No. | a (nm) | b (nm) | c (nm) | Z | Density (g/cm3) |
|---|---|---|---|---|---|---|---|---|---|
| α | tetragonal | tI12 | I42d | 122 | 0.5398 | 0.5398 | 0.64223 | 4 | 3.00 |
| β | tetragonal | tP6 | P42/nmc | 137 | 0.3696 | 0.3696 | 1.071 | 2 | 3.09 |
| γ | monoclinic | mP36 | P21/c | 14 | 0.654 | 1.131 | 1.23328 | 12 | 2.98 |
| δ | orthorhombic | oP12 | Pna21 | 33 | 0.6125 | 0.6443 | 0.7693 | 4 | 2.98 |
Here a, b, and c are lattice constants, Z is the number of structure units per unit cell, and ρ is the density calculated from the structure parameters.
The orthorhombic form (δ) rapidly changes to one of the other forms on exposure to the atmosphere. A possible explanation is that the OH− ions originating from the absorbed water facilitate the rearrangement. Rapid cooling of molten ZnCl2 gives a glass.
Molten ZnCl2 has a high viscosity at its melting point and a comparatively low electrical conductivity, which increases markedly with temperature. As indicated by a Raman scattering study, the viscosity is explained by the presence of polymers,. Neutron scattering study indicated the presence of tetrahedral ZnCl4 centers, which requires aggregation of ZnCl2 monomers as well..
In the gas phase, ZnCl2 molecules are linear with a bond length of 205 pm.
Hydrates
Various hydrates of zinc chloride are known: ZnCl2(H2O)n with n = 1, 1.33, 2.5, 3, and 4.5. However, only the 1.33-hydrate, hemipentahydrate, trihydrate, and the heminonahydrate has been structurally elucidated.
The 1.33-hydrate, previously thought to be the hemitrihydrate, consists of trans-Zn(H2O)4Cl2 centers with the chlorine atoms connected to repeating ZnCl4 chains. The hemipentahydrate, structurally formulated [Zn(H2O)5][ZnCl4], consists of Zn(H2O)5Cl octahedrons where the chlorine atom is part of a [ZnCl4]2- tetrahedera. The trihydrate consists of distinct hexaaquozinc(II) cations and tetrachlorozincate anions; formulated [Zn(H2O)6][ZnCl4]. Finally, the heminonahydrate, structurally formulated [Zn(H2O)6][ZnCl4]·3H2O also consists of distinct hexaaquozinc(II) cations and tetrachlorozincate anions like the trihydrate but has three extra water molecules.
Preparation and purification
Anhydrous ZnCl2 can be prepared from zinc and hydrogen chloride gas at 700 °C:
- Zn + 2 HCl → ZnCl2 + H2
Aqueous solutions may be readily prepared similarly by treating Zn metal, zinc carbonate, zinc oxide, and zinc sulfide with hydrochloric acid:
- ZnS + 2 HCl + 4 H2O → ZnCl2(H2O)4 + H2S
Hydrates can be produced by evaporation of an aqueous solution of zinc chloride. Different evaporation temperatures produce different hydrates; for example, evaporation at room temperature produces the 1.33-hydrate. Lower evaporation temperatures produce higher hydrates.
Commercial samples of zinc chloride typically contain water and products from hydrolysis as impurities. Such samples may be purified by recrystallization from hot dioxane. Anhydrous samples can be purified by sublimation in a stream of hydrogen chloride gas, followed by heating the sublimate to 400 °C in a stream of dry nitrogen gas. Finally, the simplest method relies on treating the zinc chloride with thionyl chloride.
Reactions
The Zn2+2 ion
Molten anhydrous ZnCl2 at 500–700 °C dissolves zinc metal, and, on rapid cooling of the melt, a yellow diamagnetic glass is formed, which Raman studies indicate contains the Zn2+2 ion.
Chloride complexes
A number of salts containing the tetrachlorozincate anion, [ZnCl4]2−, are known. "Caulton's reagent", V2Cl3(thf)6] [Zn2Cl6], which is used in organic chemistry, is an example of a salt containing [Zn2Cl6]2−. The compound Cs3ZnCl5 contains tetrahedral [ZnCl4]2− and Cl− anions, so, the compound is not caesium pentachlorozincate, but caesium tetrachlorozincate chloride. No compounds containing the [ZnCl6]4− ion (hexachlorozincate ion) have been characterized.
The compound ZnCl2·0.5HCl·H2O may be prepared by careful precipitation from a solution of ZnCl2 acidified with HCl. It contains a polymeric anion (Zn2Cl−5)n with balancing monohydrated hydronium ions, H5O+2 ions.
The coordination complex ZnCl2(NH2OH)2 (zinc dichloride di(hydroxylamine)), known as Crismer's salt, releases hydroxylamine upon heating.
Aqueous solutions of zinc chloride
Zinc chloride dissolves readily in water to give ZnClx(H2O)4−x species and some free chloride. Aqueous solutions of ZnCl2 are acidic: a 6 M aqueous solution has a pH of 1. The acidity of aqueous ZnCl2 solutions relative to solutions of other Zn2+ salts (say the sulfate) is due to the formation of the tetrahedral chloro aqua complexes where the reduction in coordination number from 6 to 4 further reduces the strength of the O–H bonds in the solvated water molecules.
In alkali solution, zinc chloride converts to various zinc hydroxychlorides. These include [Zn(OH)3Cl]2−, [Zn(OH)2Cl2]2−, [Zn(OH)Cl3]2−, and the insoluble Zn5(OH)8Cl2·H2O. The latter is the mineral simonkolleite. When zinc chloride hydrates are heated, HCl gas evolves and hydroxychlorides result.
When solutions of zinc chloride are treated with ammonia, various ammine complexes are produced. These include Zn(NH3)4Cl2·H2O and on concentration ZnCl2(NH3)2. The former contains the [Zn(NH3)6]2+ ion, and the latter is molecular with a distorted tetrahedral geometry. The species in aqueous solution have been investigated and show that [Zn(NH3)4]2+ is the main species present with [Zn(NH3)3Cl]+ also present at lower NH3:Zn ratio.
Zinc oxychloride cement
Aqueous zinc chloride reacts with zinc oxide to form an amorphous cement that was first investigated in 1855 by Stanislas Sorel. Sorel later went on to investigate the related magnesium oxychloride cement, which bears his name.
Decomposition
Anhydrous zinc chloride is able to melt and boil without any decomposition until 900 °C in an inert atmosphere. However, in the presence of oxygen, zinc chloride oxidizes to zinc oxide above 400 °C.
When hydrated zinc chloride is heated, Zn(OH)Cl is produced instead of anhydrous zinc chloride:
- ZnCl2·2H2O → Zn(OH)Cl + HCl + H2O
Cellulose dissolution in aqueous solutions of ZnCl2
Cellulose dissolves in aqueous solutions of ZnCl2, and zinc-cellulose complexes have been detected. Cellulose also dissolves in molten ZnCl2 hydrate and carboxylation and acetylation performed on the cellulose polymer.
Using zinc chloride for preparing other zinc salts
Thus, although many zinc salts have different formulas and different crystal structures, these salts behave very similarly in aqueous solution. For example, solutions prepared from any of the polymorphs of ZnCl2, as well as other halides (bromide, iodide), and the sulfate can often be used interchangeably for the preparation of other zinc compounds. Illustrative is the preparation of zinc carbonate:
Uses
In organic chemistry
Zinc chloride is used as a catalyst or reagent in diverse reactions conducted on an industrial scale. The partial hydrolysis of benzal chloride in the presence of zinc chloride is the main route to benzoyl chloride. It serves as a catalyst for the production of methylene-bis(dithiocarbamate).
The combination of hydrochloric acid and ZnCl2, known as the "Lucas reagent", is effective for the preparation of alkyl chlorides from alcohols. Similar reactions are the basis of industrial routes from methanol and ethanol respectively to methyl chloride and ethyl chloride.
Laboratory syntheses
Zinc chloride is a common reagent in the laboratory useful Lewis acid in organic chemistry.
Molten zinc chloride catalyses the conversion of methanol to hexamethylbenzene:
- 15 CH3OH → C6(CH3)6 + 3 CH4 + 15 H2O
Other examples include catalyzing (A) the Fischer indole synthesis, and also (B) Friedel-Crafts acylation reactions involving activated aromatic rings
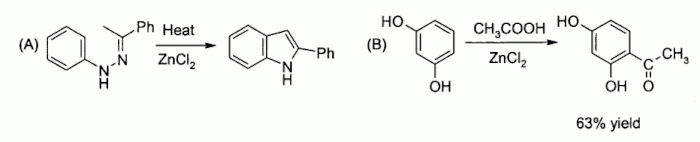
Related to the latter is the classical preparation of the dye fluorescein from phthalic anhydride and resorcinol, which involves a Friedel-Crafts acylation. This transformation has in fact been accomplished using even the hydrated ZnCl2 sample shown in the picture above.

Zinc chloride also activates benzylic and allylic halides towards substitution by weak nucleophiles such as alkenes:

In similar fashion, ZnCl2 promotes selective Na[BH3(CN)] reduction of tertiary, allylic or benzylic halides to the corresponding hydrocarbons.
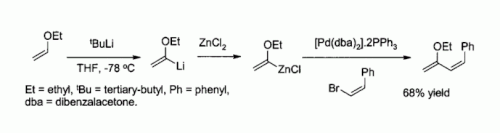
Zinc chloride is also a useful starting reagent for the synthesis of many organozinc reagents, such as those used in the palladium catalyzed Negishi coupling with aryl halides or vinyl halides. In such cases the organozinc compound is usually prepared by transmetallation from an organolithium or a Grignard reagent, for example:
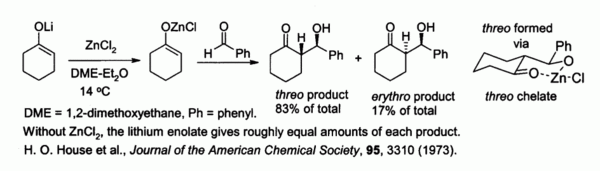
Zinc enolates, prepared from alkali metal enolates and ZnCl2, provide control of stereochemistry in aldol condensation reactions due to chelation on to the zinc. In the example shown below, the threo product was favored over the erythro by a factor of 5:1 when ZnCl2 in DME/ether was used. The chelate is more stable when the bulky phenyl group is pseudo-equatorial rather than pseudo-axial, i.e., threo rather than erythro.
As a metallurgical flux
The use of zinc chloride as a flux, sometimes in a mixture with ammonium chloride (see also Zinc ammonium chloride), involves the production of HCl and its subsequent reaction with surface oxides.
Zinc chloride reacts with metal oxides (MO) to give derivatives of the idealized formula MZnOCl2.[additional citation(s) needed] This reaction is relevant to the utility of ZnCl2 solution as a flux for soldering — it dissolves passivating oxides, exposing the clean metal surface. Fluxes with ZnCl2 as an active ingredient are sometimes called "tinner's fluid".
Zinc chloride forms two salts with ammonium chloride: [NH4]2[ZnCl4] and [NH4]3[ZnCl4]Cl, which decompose on heating liberating HCl, just as zinc chloride hydrate does. The action of zinc chloride/ammonium chloride fluxes, for example, in the hot-dip galvanizing process produces H2 gas and ammonia fumes.
In textile and paper processing
Concentrated aqueous solutions of zinc chloride (more than 64% weight/weight zinc chloride in water) are capable of dissolving starch, silk, and cellulose.
Relevant to its affinity for these materials, ZnCl2 is used as a fireproofing agent and in fabric "refresheners" such as Febreze. Vulcanized fibre is made by soaking paper in concentrated zinc chloride.
Smoke grenades
The zinc chloride smoke mixture ("HC") used in smoke grenades contains zinc oxide, hexachloroethane and granular aluminium powder, which, when ignited, react to form zinc chloride, carbon and aluminium oxide smoke, an effective smoke screen.
Fingerprint detection
Ninhydrin reacts with amino acids and amines to form a colored compound "Ruhemann's purple" (RP). Spraying with a zinc chloride solution forms a 1:1 complex RP:ZnCl(H2O)2, which is more readily detected as it fluoresces more intensely than RP.
Disinfectant and wood preservative
Dilute aqueous zinc chloride was used as a disinfectant under the name "Burnett's Disinfecting Fluid". From 1839 Sir William Burnett promoted its use as a disinfectant as well as a wood preservative. The Royal Navy conducted trials into its use as a disinfectant in the late 1840s, including during the cholera epidemic of 1849; and at the same time experiments were conducted into its preservative properties as applicable to the shipbuilding and railway industries. Burnett had some commercial success with his eponymous fluid. Following his death however, its use was largely superseded by that of carbolic acid and other proprietary products.
Natural occurrence
Anhydrous zinc chloride or its hydrates is not known in nature. However, the related zinc chloride hydroxide monohydrate is known as simonkolleite in nature.
Safety
Zinc chloride is a chemical irritant of the eyes, skin, and respiratory system.
References
This article uses material from the Wikipedia English article Zinc chloride, which is released under the Creative Commons Attribution-ShareAlike 3.0 license ("CC BY-SA 3.0"); additional terms may apply (view authors). Content is available under CC BY-SA 4.0 unless otherwise noted. Images, videos and audio are available under their respective licenses.
®Wikipedia is a registered trademark of the Wiki Foundation, Inc. Wiki English (DUHOCTRUNGQUOC.VN) is an independent company and has no affiliation with Wiki Foundation.
