Helium: Chemical element with symbol He and atomic number 2; rare gas
Helium is a chemical element.
It has the chemical symbol He, atomic number 2, and atomic weight of about 4.002602. There are 9 isotopes of helium, only two of which are stable. These are 3He and 4He. 4He is by far the most common isotope.
 | |||||||||||||||||||||
| Helium | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronunciation | /ˈhiːliəm/ | ||||||||||||||||||||
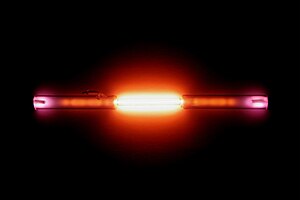
| Appearance | colorless gas, exhibiting a red-orange glow when placed in an electric field | ||||||||||||||||||||
| Standard atomic weight Ar°(He) | |||||||||||||||||||||
| 4.002602(2) | |||||||||||||||||||||
| Helium in the periodic table | |||||||||||||||||||||
| |||||||||||||||||||||
| Group | group 18 (noble gases) | ||||||||||||||||||||
| Period | period 1 | ||||||||||||||||||||
| Block | s-block | ||||||||||||||||||||
| Electron configuration | 1s2 | ||||||||||||||||||||
| Electrons per shell | 2 | ||||||||||||||||||||
| Physical properties | |||||||||||||||||||||
| Phase at STP | gas | ||||||||||||||||||||
| Melting point | 0.95 K (−272.20 °C, −457.96 °F) (at 2.5 MPa) | ||||||||||||||||||||
| Boiling point | 4.222 K (−268.928 °C, −452.070 °F) | ||||||||||||||||||||
| Density (at STP) | 0.1786 g/L | ||||||||||||||||||||
| when liquid (at m.p.) | 0.145 g/cm3 | ||||||||||||||||||||
| when liquid (at b.p.) | 0.125 g/cm3 | ||||||||||||||||||||
| Triple point | 2.177 K, 5.043 kPa | ||||||||||||||||||||
| Critical point | 5.1953 K, 0.22746 MPa | ||||||||||||||||||||
| Heat of fusion | 0.0138 kJ/mol | ||||||||||||||||||||
| Heat of vaporization | 0.0829 kJ/mol | ||||||||||||||||||||
| Molar heat capacity | 20.78 J/(mol·K) | ||||||||||||||||||||
Vapor pressure (defined by ITS-90)
| |||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||
| Oxidation states | 0 | ||||||||||||||||||||
| Electronegativity | Pauling scale: no data | ||||||||||||||||||||
| Ionization energies |
| ||||||||||||||||||||
| Covalent radius | 28 pm | ||||||||||||||||||||
| Van der Waals radius | 140 pm | ||||||||||||||||||||
| Other properties | |||||||||||||||||||||
| Natural occurrence | primordial | ||||||||||||||||||||
| Crystal structure | hexagonal close-packed (hcp) | ||||||||||||||||||||
| Speed of sound | 972 m/s | ||||||||||||||||||||
| Thermal conductivity | 0.1513 W/(m⋅K) | ||||||||||||||||||||
| Magnetic ordering | diamagnetic | ||||||||||||||||||||
| Molar magnetic susceptibility | −1.88·10−6 cm3/mol (298 K) | ||||||||||||||||||||
| CAS Number | 7440-59-7 | ||||||||||||||||||||
| History | |||||||||||||||||||||
| Naming | after Helios, Greek Titan of the Sun | ||||||||||||||||||||
| Discovery | Pierre Janssen, Norman Lockyer (1868) | ||||||||||||||||||||
| First isolation | William Ramsay, Per Teodor Cleve, Abraham Langlet (1895) | ||||||||||||||||||||
| Isotopes of helium | |||||||||||||||||||||
| |||||||||||||||||||||

Helium is called a noble gas, because it does not regularly mix with other chemicals and form new compounds. It has the lowest boiling point of all the elements. It is the second most common element in the universe, after hydrogen, and has no color or smell. However, helium has a red-orange glow when placed in an electric field. Helium does not usually react with anything else. Astronomers detected the presence of helium in 1868, when its spectrum was identified in light from the Sun. This was before its discovery on Earth.
Helium is used to fill balloons and airships because its density is lighter than air. It does not burn, so is safe for that kind of use. It is also used in some kinds of light bulbs. People can breathe in helium: It makes their voices sound higher than it normally does. This may seem silly, but it can actually be quite dangerous as if they breathe in too much, hypoxia can injure or kill them as they are not breathing normal air. Breathing too much helium can also cause long-term effects to vocal cords.
Helium is created through the process of nuclear fusion in the Sun, and in similar stars. During this process, four hydrogen atoms are fused together to form one helium atom. On Earth it is made by the natural radioactive decay of heavy radioactive elements like thorium and uranium, although there are other examples. The alpha particles emitted by such decays consist of helium-4 nuclei.
History
Helium was discovered by the French astronomer pierre Janssen on August 18, 1868, as a bright yellow line in the spectrum of the chromosphere of the Sun. The line was thought to be sodium. On the same year, English astronomer, Norman Lockyer, also observed it and found that it was caused by a new element. Lockyer and English chemist Edward Frankland named the element helium, from the Greek word for the Sun, ἥλιος (helios).
Characteristics
Helium is the second least reactive noble gas after neon. It is the second least reactive of all elements. It is chemically inert and monatomic in all standard conditions. Helium is the least water-soluble monatomic gas.
Uses
Helium is used as a shielding gas in growing silicon and germanium crystals, in making titanium and zirconium, and in gas chromatography, because it is inert. Helium is used as a shielding gas in arc welding.
Helium is mixed with oxygen and other gases for deep underwater diving because it does not cause nitrogen narcosis.
Helium is also used to condense hydrogen and oxygen to make rocket fuel. It is used to remove the fuel and oxidizer from ground support equipment before the rocket launches. It is used to cool liquid hydrogen in space vehicles before the rocket launches.
Helium is used as a heat-transfer medium in some nuclear reactors that are cooled down by gas. Helium is also used in some hard disk drives. Helium at low temperatures is used in cryogenics.
Supply
Helium has become rare on Earth. If it gets free into the air it leaves the planet. Unlike hydrogen, which reacts with oxygen to form water, helium is not reactive. It stays as a gas. For many years after the 1925 Helium Act, the USA collected helium in a National Helium Reserve. American helium comes from wells in the Great Plains area. At present, more helium is supplied by Qatar than by the USA.
Several research organisations have released statements on the scarcity and conservation of helium. These organisations released policy recommendations as early as 1995 and as late as 2016 urging the United States government to store and conserve helium because of the natural limits to the helium supply and the unique nature of the element. For researchers, helium is irreplaceable because it is essential for producing very low temperatures. Helium at low temperatures is used in cryogenics, and in certain cryogenics applications. Liquid helium is used to cool certain metals to the extremely low temperatures required for superconductivity, such as in superconducting magnets for magnetic resonance imaging.
References
This article uses material from the Wikipedia Simple English article Helium, which is released under the Creative Commons Attribution-ShareAlike 3.0 license ("CC BY-SA 3.0"); additional terms may apply (view authors). Content is available under CC BY-SA 4.0 unless otherwise noted. Images, videos and audio are available under their respective licenses.
®Wikipedia is a registered trademark of the Wiki Foundation, Inc. Wiki Simple English (DUHOCTRUNGQUOC.VN) is an independent company and has no affiliation with Wiki Foundation.
