Monograph United States Food and Drug Administration
Monograph United States Food and Drug Administration - Search results - Wiki Monograph United States Food And Drug Administration
The page "Monograph+United+States+Food+and+Drug+Administration" does not exist. You can create a draft and submit it for review or request that a redirect be created, but consider checking the search results below to see whether the topic is already covered.
 The United States Food and Drug Administration (FDA or US FDA) is a federal agency of the Department of Health and Human Services. The FDA is responsible...
The United States Food and Drug Administration (FDA or US FDA) is a federal agency of the Department of Health and Human Services. The FDA is responsible...- from the Federal Register: "The Food and Drug Administration (FDA) is issuing a final rule in the form of a final monograph establishing conditions under...
 the United States, the manufacture and sale of OTC substances are regulated by the Food and Drug Administration. The FDA requires that all "new drugs" obtain...
the United States, the manufacture and sale of OTC substances are regulated by the Food and Drug Administration. The FDA requires that all "new drugs" obtain...- the responsibility of the U.S. Food and Drug Administration (FDA) and other government authorities in the United States.[vague][citation needed] The U...
- Prescription drug list prices in the United States continually are among the highest in the world. The high cost of prescription drugs became a major...
 United States Food and Drug Administration. 8 February 2019. "Expiration Dates Matter". United States Food and Drug Administration. 14 July 2015. Retrieved...
United States Food and Drug Administration. 8 February 2019. "Expiration Dates Matter". United States Food and Drug Administration. 14 July 2015. Retrieved... The United States Food and Drug Administration Modernization Act of 1997 (FDAMA) amended the Federal Food, Drug, and Cosmetic Act. This act is related...
The United States Food and Drug Administration Modernization Act of 1997 (FDAMA) amended the Federal Food, Drug, and Cosmetic Act. This act is related...- Aducanumab (category Drugs with non-standard legal status)Biogen and Eisai. Aducanumab is given via intravenous infusion. Aducanumab was approved for medical use in the United States by the Food and Drug Administration...
 Apixaban (category Drugs with non-standard legal status)new drug application (NDA) for the approval of apixaban was submitted to the U.S. Food and Drug Administration (FDA) by Bristol-Myers Squibb (BMS) and Pfizer...
Apixaban (category Drugs with non-standard legal status)new drug application (NDA) for the approval of apixaban was submitted to the U.S. Food and Drug Administration (FDA) by Bristol-Myers Squibb (BMS) and Pfizer... Semaglutide (category Drugs with non-standard legal status)original weight within 5 years of stopping treatment. The US Food and Drug Administration (FDA) approved semaglutide based on evidence from seven clinical...
Semaglutide (category Drugs with non-standard legal status)original weight within 5 years of stopping treatment. The US Food and Drug Administration (FDA) approved semaglutide based on evidence from seven clinical... Dietary supplement (redirect from Food supplements)Food and Drug Administration (FDA) is governed by various statutes enacted by the United States Congress. Pursuant to the Federal Food, Drug, and Cosmetic...
Dietary supplement (redirect from Food supplements)Food and Drug Administration (FDA) is governed by various statutes enacted by the United States Congress. Pursuant to the Federal Food, Drug, and Cosmetic...- Empagliflozin (category Drugs developed by Eli Lilly and Company)the United States, with more than 8 million prescriptions. It has received approval as a generic medication from the US Food and Drug Administration (FDA)...
- U.S. Food and Drug Administration (FDA), AHFS, Harvard Health Publications, Mayo Clinic, and Animalytics (a veterinary products database). Drugs.com is...
 Triamcinolone acetonide (category Drugs with non-standard legal status)2014, the U.S. Food and Drug Administration (FDA) made triamcinolone acetonide an over-the-counter drug in the United States in nasal spray form under the...
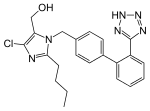
Triamcinolone acetonide (category Drugs with non-standard legal status)2014, the U.S. Food and Drug Administration (FDA) made triamcinolone acetonide an over-the-counter drug in the United States in nasal spray form under the...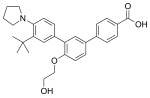 Trifarotene (category Dermatologic drug stubs)was granted orphan drug designation for the treatment of congenital ichthyosis by both the U.S. Food and Drug Administration (FDA) and the European Medicines...
Trifarotene (category Dermatologic drug stubs)was granted orphan drug designation for the treatment of congenital ichthyosis by both the U.S. Food and Drug Administration (FDA) and the European Medicines...- Adalimumab (category Drugs with non-standard legal status)Adalimumab may be effective and well tolerated in ulcerative colitis. It was approved by the US Food and Drug Administration (FDA) for treatment of moderate...
 Ibuprofen (redirect from Burana (drug))by the Food and Drug Administration (FDA) for closure of patent ductus arteriosus in premature infants weighing between 500 and 1,500 g (1 and 3 lb),...
Ibuprofen (redirect from Burana (drug))by the Food and Drug Administration (FDA) for closure of patent ductus arteriosus in premature infants weighing between 500 and 1,500 g (1 and 3 lb),...- Talquetamab (category Drugs with non-standard legal status)infection, and diarrhea. Talquetamab was approved for medical use in both the United States and the European Union in August 2023. The US Food and Drug Administration...
 Losartan (category Drugs with non-standard legal status)October 2014, the U.S. Food and Drug Administration (FDA) issued a black box warning that losartan can cause fetal toxicity, and should be discontinued...
Losartan (category Drugs with non-standard legal status)October 2014, the U.S. Food and Drug Administration (FDA) issued a black box warning that losartan can cause fetal toxicity, and should be discontinued... Sparsentan (category Drugs not assigned an ATC code)and angiotensin II receptor antagonist. It is taken by mouth. It was approved for medical use in the United States in February 2023. The US Food and Drug...
Sparsentan (category Drugs not assigned an ATC code)and angiotensin II receptor antagonist. It is taken by mouth. It was approved for medical use in the United States in February 2023. The US Food and Drug...
- Public Law 116-136 (redirect from Coronavirus Aid, Relief, and Economic Security Act)Food and Drug Administration's Field Accomplishments and Compliance Tracking System (FACTS) (or any successor system). "(5) The term 'OTC monograph drug'
- outcomes at 57 to 63 days LMP and 64 to 70 days LMP. Updating clinical protocols and revising the Food and Drug administration label for Mifeprex® to change
- the United States Drug Enforcement Administration (DEA), which investigates the illegal production of controlled substances on an interstate and international











