Lead–Acid Battery Electrochemistry
Lead–Acid Battery Electrochemistry - Search results - Wiki Lead–Acid Battery Electrochemistry
The page "Lead–Acid+Battery+Electrochemistry" does not exist. You can create a draft and submit it for review or request that a redirect be created, but consider checking the search results below to see whether the topic is already covered.
 The lead-acid battery is a type of rechargeable battery first invented in 1859 by French physicist Gaston Planté. It is the first type of rechargeable...
The lead-acid battery is a type of rechargeable battery first invented in 1859 by French physicist Gaston Planté. It is the first type of rechargeable...- consumed, they cannot be electrically recharged. The development of the lead-acid battery and subsequent "secondary" or "chargeable" types allowed energy to...
 Electrochemistry is the branch of physical chemistry concerned with the relationship between electrical potential difference and identifiable chemical...
Electrochemistry is the branch of physical chemistry concerned with the relationship between electrical potential difference and identifiable chemical... several important applications in electrochemistry, in particular as the positive plate of lead acid batteries. Lead dioxide has two major polymorphs,...
several important applications in electrochemistry, in particular as the positive plate of lead acid batteries. Lead dioxide has two major polymorphs,... include the lead–acid batteries used in vehicles and lithium-ion batteries used for portable electronics such as laptops and mobile phones. Batteries come in...
include the lead–acid batteries used in vehicles and lithium-ion batteries used for portable electronics such as laptops and mobile phones. Batteries come in... increased to about half of that of primary batteries, and significantly greater than lead–acid batteries. Jungner experimented with substituting iron...
increased to about half of that of primary batteries, and significantly greater than lead–acid batteries. Jungner experimented with substituting iron... established rechargeable battery technologies in the market currently: the lithium-ion battery and the rechargeable lead–acid battery. Companies around the...
established rechargeable battery technologies in the market currently: the lithium-ion battery and the rechargeable lead–acid battery. Companies around the...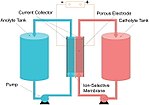 than to conventional batteries (such as lead–acid or lithium-ion). The main reason fuel cells are not considered to be batteries, is because originally...
than to conventional batteries (such as lead–acid or lithium-ion). The main reason fuel cells are not considered to be batteries, is because originally... the nickel–iron design to be, "far superior to batteries using lead plates and acid" (lead–acid battery). Edison had several patents: U.S. patent 678,722/1901...
the nickel–iron design to be, "far superior to batteries using lead plates and acid" (lead–acid battery). Edison had several patents: U.S. patent 678,722/1901... A lemon battery is a simple battery often made for the purpose of education. Typically, a piece of zinc metal (such as a galvanized nail) and a piece...
A lemon battery is a simple battery often made for the purpose of education. Typically, a piece of zinc metal (such as a galvanized nail) and a piece... and Interfacial Electrochemistry. 72: 1–31. doi:10.1016/S0022-0728(76)80072-1. Yoshino, A., Sanechika, K. & Nakajima, T. Secondary battery. JP patent 1989293...
and Interfacial Electrochemistry. 72: 1–31. doi:10.1016/S0022-0728(76)80072-1. Yoshino, A., Sanechika, K. & Nakajima, T. Secondary battery. JP patent 1989293...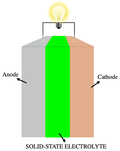 electric vehicles use a variety of battery technologies, including lithium ion (Li-ion), nickel–metal hydride (NiMH), lead–acid, and electric double-layer capacitor...
electric vehicles use a variety of battery technologies, including lithium ion (Li-ion), nickel–metal hydride (NiMH), lead–acid, and electric double-layer capacitor... solution of the purchased HNO3 mixed with Type 1 DI Water. In electrochemistry, nitric acid is used as a chemical doping agent for organic semiconductors...
solution of the purchased HNO3 mixed with Type 1 DI Water. In electrochemistry, nitric acid is used as a chemical doping agent for organic semiconductors... Galvanic cell (redirect from Voltaic battery)A battery is a set of galvanic cells that are connected together to form a single source of voltage. For instance, a typical 12V lead–acid battery has...
Galvanic cell (redirect from Voltaic battery)A battery is a set of galvanic cells that are connected together to form a single source of voltage. For instance, a typical 12V lead–acid battery has... right). The penny battery experiment is common during electrochemistry units in an educational setting. Each cell in a penny battery can produce up to...
right). The penny battery experiment is common during electrochemistry units in an educational setting. Each cell in a penny battery can produce up to...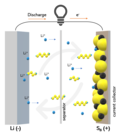 scooter battery system primarily for the Chinese market, which had a capacity of 1.2 kWh using 10 Ah Long Life cells, and weighed 60% less than lead acid batteries...
scooter battery system primarily for the Chinese market, which had a capacity of 1.2 kWh using 10 Ah Long Life cells, and weighed 60% less than lead acid batteries... Jelly roll Lead–acid battery List of battery sizes List of battery types Lithium-ion battery Lithium iron phosphate battery Nickel–zinc battery Nickel(II)...
Jelly roll Lead–acid battery List of battery sizes List of battery types Lithium-ion battery Lithium iron phosphate battery Nickel–zinc battery Nickel(II)... Electrode (redirect from Battery electrode)recharged. The first was the lead–acid battery, invented in 1859 by French physicist Gaston Planté. This type of battery is still the most widely used...
Electrode (redirect from Battery electrode)recharged. The first was the lead–acid battery, invented in 1859 by French physicist Gaston Planté. This type of battery is still the most widely used...- The Iron Redox Flow Battery (IRFB), also known as Iron Salt Battery (ISB), stores and releases energy through the electrochemical reaction of iron salt...
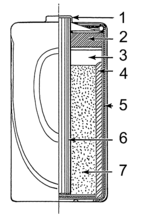 Dry cell (redirect from Dry battery)fragile glass containers with lead rods hanging from the open top and needed careful handling to avoid spillage. Lead–acid batteries did not achieve the safety...
Dry cell (redirect from Dry battery)fragile glass containers with lead rods hanging from the open top and needed careful handling to avoid spillage. Lead–acid batteries did not achieve the safety...
- oxidation at the anode (see Electrochemistry). It is possible to distinguish between double salts and salts of compound acids. Thus J. W. Hittorf showed
- of lead-acid batteries, its structure and principles have not changed substantially because of electrochemistry. Benefits to lead-acid batteries, rechargeable


















