ᱦᱟᱭᱤᱰᱨᱚᱡᱟᱱ ᱥᱟᱞᱯᱷᱟᱭᱤᱰ
ᱦᱟᱭᱰᱨᱚᱡᱟᱱ ᱥᱟᱞᱯᱷᱟᱭᱤᱰ ᱢᱤᱫ ᱨᱟᱥᱟᱭᱱᱤᱠ ᱭᱚᱣᱜᱤᱠ ᱥᱟᱶ ᱯᱷᱚᱨᱢᱩᱞᱟ H2S ᱠᱟᱱᱟ᱾ H2S ᱫᱚ ᱵᱷᱚᱞᱠᱟᱱᱤᱠ ᱜᱮᱥ, ᱱᱮᱪᱩᱨᱟᱞ ᱜᱮᱥ ᱟᱨ ᱫᱟᱜ ᱨᱮᱱᱟᱜ ᱯᱷᱮᱰᱟᱛ ᱠᱷᱚᱱ ᱧᱟᱢᱚᱜᱼᱟ ᱾ ᱢᱟᱹᱱᱢᱤᱭᱟᱜ ᱦᱚᱲᱢᱚ ᱦᱚᱸ ᱱᱟᱥᱮ ᱩᱰᱤᱡ H2S ᱮ ᱛᱮᱭᱟᱨᱮᱫᱼᱟ ᱟᱨ ᱥᱤᱜᱽᱱᱟᱞᱤᱝ ᱢᱚᱞᱤᱠᱩᱞ (signaling molecule) ᱞᱮᱠᱟᱛᱮ ᱵᱮᱵᱷᱟᱨᱮᱫᱼᱟ ᱾
 | |||
| |||
| Names | |||
|---|---|---|---|
| ᱥᱤᱥᱴᱟᱢᱮᱴᱤᱠ IUPAC ᱧᱩᱛᱩᱢ Hydrogen sulfide | |||
Other names
| |||
| Identifiers | |||
CAS Number | |||
3D model (JSmol) | |||
| 3DMet | B01206 Archived ᱒᱐᱑᱙-᱐᱔-᱐᱗ at the Wayback Machine. | ||
Beilstein Reference | 3535004 | ||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.029.070 | ||
| EC Number | 231-977-3 | ||
Gmelin Reference | 303 | ||
| KEGG | |||
| MeSH | Hydrogen+sulfide | ||
PubChem CID | |||
| RTECS number | MX1225000 | ||
| UNII |
| ||
| UN number | 1053 | ||
CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
Chemical formula | H2S | ||
| Molar mass | 34.08 g·mol−1 | ||
| Appearance | Colorless gas | ||
| Odor | Pungent, like that of rotten eggs | ||
| Density | 1.363 g dm−3 | ||
| Melting point | −82 °C (−116 °F; 191 K) | ||
| Boiling point | −60 °C (−76 °F; 213 K) | ||
Solubility in water | 4 g dm−3 (at 20 °C) | ||
| Vapor pressure | 1740 kPa (at 21 °C) | ||
| Acidity (pKa) | 7.0 | ||
| Conjugate acid | Sulfonium | ||
| Conjugate base | Bisulfide | ||
Magnetic susceptibility (χ) | −25.5·10−6 cm3/mol | ||
Refractive index (nD) | 1.000644 (0 °C) | ||
| Structure | |||
Point group | C2v | ||
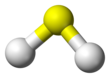
Molecular shape | Bent | ||
Dipole moment | 0.97 D | ||
| Thermochemistry | |||
Heat capacity (C) | 1.003 J K−1 g−1 | ||
Std molar entropy (S | 206 J mol−1 K−1 | ||
Std enthalpy of formation (ΔfH⦵298) | −21 kJ mol−1 | ||
| Hazards | |||
| Main hazards | Flammable and highly toxic | ||
EU classification (DSD) (outdated) | |||
| R-phrases (outdated) | R12, R26, R50 | ||
| S-phrases (outdated) | (S1/2), S9, S16, S36, S38, S45, S61 | ||
| NFPA 704 |  4 4 0 | ||
| Flash point | −82.4 °C (−116.3 °F; 190.8 K) | ||
Autoignition temperature | 232 °C (450 °F; 505 K) | ||
| Explosive limits | 4.3–46% | ||
| Lethal dose or concentration (LD, LC): | |||
LC50 (median concentration) |
| ||
LCLo (lowest published) |
| ||
| US health exposure limits (NIOSH): | |||
PEL (Permissible) | C 20 ppm; 50 ppm [10-minute maximum peak] | ||
REL (Recommended) | C 10 ppm (15 mg/m3) [10-minute] | ||
IDLH (Immediate danger) | 100 ppm | ||
| Related compounds | |||
Related hydrogen chalcogenides |
| ||
Related compounds | Phosphine | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
ᱱᱚᱶᱟ ᱫᱚ ᱵᱮᱨᱚᱝ ᱪᱟᱞᱠᱚᱡᱮᱱ ᱦᱟᱭᱰᱨᱟᱭᱤᱰ (chalcogen hydride) ᱜᱮᱥ ᱥᱟᱶᱛᱮ ᱥᱮᱭᱟ ᱥᱤᱢᱵᱤᱞᱤ ᱞᱮᱠᱟ ᱥᱚ ᱣᱟᱱᱟ ᱾ ᱱᱚᱶᱟ ᱫᱚ ᱟᱹᱰᱤ ᱵᱤᱥ ᱜᱮᱭᱟ, ᱠᱨᱚᱡᱤᱵᱽ ᱟᱨ ᱞᱟᱜᱮ ᱟᱛᱟᱨᱚᱜᱼᱟ ᱾
ᱥᱩᱭᱰᱤᱥ ᱨᱤᱱᱤᱡ ᱥᱟᱬᱮᱥᱤᱭᱟᱹ ᱠᱟᱨᱞ ᱣᱤᱞᱦᱮᱞᱢ ᱥᱮᱞᱮ (Carl Wilhelm Scheele) ᱱᱚᱶᱟ ᱠᱮᱢᱤᱠᱟᱞ ᱠᱚᱢᱯᱚᱡᱤᱥᱚᱱ ᱫᱚᱭ ᱧᱟᱢ ᱚᱰᱚᱠ ᱞᱮᱫ ᱛᱟᱦᱮᱸᱫ ᱑᱗᱗᱗ ᱥᱟᱞᱮ ᱨᱮ ᱾
ᱜᱩᱱ
ᱥᱤᱨᱡᱟᱹᱣ
- FeS + 2 HCl → FeCl2 + H2S
- ᱱᱤᱴᱨᱮᱴ ᱢᱮᱥᱟ
- ᱠᱟᱞᱥᱤᱭᱚᱢ ᱱᱟᱭᱴᱨᱮ ᱫᱚ ᱞᱤᱸᱡᱤᱱ ᱫᱟᱜ ᱨᱮ ᱦᱟᱭᱰᱨᱚᱜᱮᱱ ᱥᱚᱞᱯᱷᱟᱭᱤᱰ ᱛᱮᱭᱟᱨᱚᱜ ᱠᱷᱚᱱ ᱵᱟᱧᱪᱟᱣ ᱞᱟᱹᱜᱤᱫ ᱵᱮᱵᱷᱟᱨ ᱜᱟᱱᱚᱜᱼᱟ ᱾
ᱯᱷᱩᱭᱤᱞ ᱜᱮᱥ ᱠᱷᱚᱱ ᱚᱪᱚᱜ
ᱱᱚᱸᱰᱮ ᱦᱚᱸ ᱧᱮᱞᱢᱮ
ᱥᱟᱹᱠᱷᱭᱟᱹᱛ
This article uses material from the Wikipedia ᱥᱟᱱᱛᱟᱲᱤ article ᱦᱟᱭᱤᱰᱨᱚᱡᱟᱱ ᱥᱟᱞᱯᱷᱟᱭᱤᱰ, which is released under the Creative Commons Attribution-ShareAlike 3.0 license ("CC BY-SA 3.0"); additional terms may apply (view authors). Content is available under CC BY-SA 4.0 unless otherwise noted. Images, videos and audio are available under their respective licenses.
®Wikipedia is a registered trademark of the Wiki Foundation, Inc. Wiki ᱥᱟᱱᱛᱟᱲᱤ (DUHOCTRUNGQUOC.VN) is an independent company and has no affiliation with Wiki Foundation.