Natural Nuclear Fission Reactor
A natural nuclear fission reactor is a uranium deposit where self-sustaining nuclear chain reactions occur.
This article includes a list of general references, but it lacks sufficient corresponding inline citations. (August 2022) |
The conditions under which a natural nuclear reactor could exist were predicted in 1956 by Paul Kuroda. The remnants of an extinct or fossil nuclear fission reactor, where self-sustaining nuclear reactions have occurred in the past, are verified by analysis of isotope ratios of uranium and of the fission products (and the stable daughter nuclides of those fission products). This was first discovered in 1972 in Oklo, Gabon by researchers from French Commissariat à l'énergie atomique (CEA) under conditions very similar to Kuroda's predictions.
Oklo is the only location where this phenomenon is known to have occurred, and consists of 16 sites with patches of centimeter-sized ore layers. There, self-sustaining nuclear fission reactions are thought to have taken place approximately 1.7 billion years ago, during the Statherian period of the Paleoproterozoic, and continued for a few hundred thousand years, probably averaging less than 100 kW of thermal power during that time.
Gabon was a French colony when the first analyses of the subsoil were carried out by the CEA from the MABA base in Franceville, more precisely by its industrial direction which later became COGEMA, leading in 1956 to the discovery of uranium deposits in the region.[citation needed][clarification needed]
France almost immediately opened mines, managed by the “Compagnie des Mines d'Uranium de Franceville” (COMUF), to exploit the resources, near the village of Mounana. After independence in 1960, the state of Gabon received a small share of the company's profits.
The "Oklo phenomenon" was discovered in June 1972 by the laboratory at the uranium enrichment plant in Pierrelatte, France. Routine analysis of a sample of natural uranium revealed a slight but abnormal deficit of uranium 235 (235U)6. The normal proportion of 235U is 0.7202%, whereas this sample showed only 0.7171%. As the quantities of fissile isotopes are precisely catalogued, this discrepancy had to be explained, so an investigation was launched by CEA on samples from all the mines operated by CEA in France, Gabon and Niger, and at all stages of ore processing and uranium purification.
For uranium and 235U analyses, CEA's Production Division relies on the Analytical Laboratory at the Pierrelatte plant and on CEA's Central Analysis and Control Laboratory at Cadarache, headed by Michele Neuilly, where Jean François Dozol is in charge of mass spectrometry analyses.
Discovery of the Oklo fossil reactors
Analyses carried out at Pierrelatte and Cadarache showed that magnesium uranates (or yellow cakes) from Gabon had a variable but constant 235U depletion. On July 7, 1972, researchers at Cadarache discovered an anomaly in uranium ore from Oklo in Gabon. Its 235U content was much lower than usual. Isotopic analyses revealed the origin of the 235U depletion: the depleted uranium came from Oklo ore in Gabon, mined by COMUF. A systematic analysis campaign was then carried out in the Cadarache and Pierrelatte laboratories (uranium content measurements, isotopic content measurements). On Oklo samples, Cadarache analysts noted a 235U depletion for magnesium uranate from the Mounana plant (235U = 0.625%) and an even greater depletion for a magnesium uranate (Oklo M) (235U = 0.440%): Oklo 310 and 311 ores have uranium contents of 12% and 46% respectively, and 235U contents of 0.592% and 0.625%.
In this context, J.F. Dozol took the initiative of analyzing magnesium uranate and ore samples from Oklo on the AEI MS 702 Spark Source Mass Spectrometer (SSMS).
The advantage of the SSMS is its ability to produce substantial quantities of ions from all the elements present in the electrodes. The electrodes, between which a spark is generated, have to be conductive (to achieve this, Oklo samples were mixed with high-purity silver). All the isotopes in the sample, from lithium to uranium, are plotted on a photo plate. On examining the plate (see below), J.F. Dozol noted in particular the very high uranium content of Oklo 311 ore:
- elements present in significant quantities around masses 85-105 and 130-150, corresponding to the two bumps of 235U fission yields. (The mass distribution of fission products follows a "camel's hump" curve, with two maxima),
- the last lanthanides (holmium to lutetium) are not detected (beyond mass166). In nature, all 14 lanthanides are found; in nuclear fuel, having undergone fission reactions, the isotopes of the last lanthanides are not detected.

The next step is isotopic analysis of certain elements on a thermal ionization mass spectrometer, after chemical separation of neodymium and samarium. From the first analyses of Oklo "M" uranate and "Oklo 311" ore, it is clear that neodymium and samarium have an isotopic composition much closer to that found in irradiated fuel than to that of the natural element. The detection of 142Nd and 144Sm isotopes not produced by fission indicates that these elements are also present in the natural state, from which their contribution can be subtracted.
These results were passed on to neutron scientist Jean Claude Nimal (CEA Saclay), who estimated the neutron flux received by the analyzed sample on the basis of its 235U deficit. This made it possible to estimate the neutron capture by the isotopes 143Nd and 145Nd, leading to the additional formation of 144Nd and 146Nd respectively. This excess must be subtracted to obtain fission yields for uranium 235. As can be seen from the table below, the fission yields (M) agree with the results corrected (C) for the presence of natural neodymium and neutron capture.
| Nd | 143 | 144 | 145 | 146 | 148 | 150 |
|---|---|---|---|---|---|---|
| C/M | 0.99 | 1.00 | 1.00 | 1.01 | 0.98 | 1.06 |
Fission product isotope signatures
U
which had been subjected to thermal neutrons.
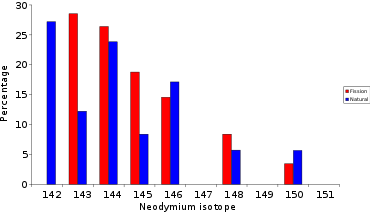
Neodymium
The neodymium found at Oklo has a different isotopic composition to that of natural neodymium: the latter contains 27% 142Nd, while that of Oklo contains less than 6%. The 142Ndis not produced by fission; the ore contains both fission-produced and natural neodymium. From this 142Ndcontent, we can subtract the natural neodymium and gain access to the isotopic composition of neodymium produced by the fission of 235U. The two isotopes 143Ndand 145Ndlead to the formation of 144Ndand 146Ndby neutron capture, and this excess must be corrected (see above) to obtain perfect agreement between this corrected isotopic composition and that deduced from fission yields.
Ruthenium

U
which had been subjected to thermal neutrons. The 100
Mo
(an extremely long-lived double beta emitter) has not had time to decay to 100
Ru
in more than trace quantities over the time since the reactors stopped working.
Similar investigations into the isotopic ratios of ruthenium at Oklo found a much higher 99
Ru
concentration than otherwise naturally occurring (27–30% vs. 12.7%). This anomaly could be explained by the decay of 99
Tc
to 99
Ru
. In the bar chart the normal natural isotope signature of ruthenium is compared with that for fission product ruthenium which is the result of the fission of 235
U
with thermal neutrons. It is clear that the fission ruthenium has a different isotope signature. The level of 100
Ru
in the fission product mixture is low because fission produces neutron rich isotopes which subsequently beta decay and 100
Ru
would only be produced in appreciable quantities by double beta decay of the very long-lived (half life 7.1×1018 years) molybdenum isotope 100
Mo. On the timescale of when the reactors were in operation, very little (about 0.17 ppb) decay to 100
Ru
will have occurred. Other pathways of 100
Ru production like neutron capture in 99
Ru or 99
Tc (quickly followed by beta decay) can only have occurred during high neutron flux and thus ceased when the fission chain reaction stopped.
Mechanism
The natural nuclear reactor at Oklo formed when a uranium-rich mineral deposit became inundated with groundwater, which could act as a moderator for the neutrons produced by nuclear fission. A chain reaction took place, producing heat that caused the groundwater to boil away; without a moderator that could slow the neutrons, however, the reaction slowed or stopped. The reactor thus had a negative void coefficient of reactivity, something employed as a safety mechanism in human-made light water reactors. After cooling of the mineral deposit, the water returned, and the reaction restarted, completing a full cycle every 3 hours. The fission reaction cycles continued for hundreds of thousands of years and ended when the ever-decreasing fissile materials, coupled with the build-up of neutron poisons, no longer could sustain a chain reaction.
Fission of uranium normally produces five known isotopes of the fission-product gas xenon; all five have been found trapped in the remnants of the natural reactor, in varying concentrations. The concentrations of xenon isotopes, found trapped in mineral formations 2 billion years later, make it possible to calculate the specific time intervals of reactor operation: approximately 30 minutes of criticality followed by 2 hours and 30 minutes of cooling down (exponentially decreasing residual decay heat) to complete a 3-hour cycle. Xenon-135 is the strongest known neutron poison. However, it is not produced directly in appreciable amounts but rather as a decay product of Iodine-135 (or one of its parent nuclides). Xenon-135 itself is unstable and decays to caesium-135 if not allowed to absorb neutrons. While caesium-135 is relatively long lived, all caesium-135 produced by the Oklo reactor has since decayed further to stable barium-135. Meanwhile, Xenon-136, the product of neutron capture in xenon-135 only decays extremely slowly via double beta decay and thus scientists were able to determine the neutronics of this reactor by calculations based on those isotope ratios almost two billion years after it stopped fissioning uranium.

A key factor that made the reaction possible was that, at the time the reactor went critical 1.7 billion years ago, the fissile isotope 235
U
made up about 3.1% of the natural uranium, which is comparable to the amount used in some of today's reactors. (The remaining 96.9% was non-fissile 238
U
and roughly 55 ppm 234
U.) Because 235
U
has a shorter half-life than 238
U
, and thus decays more rapidly, the current abundance of 235
U
in natural uranium is only 0.72%. A natural nuclear reactor is therefore no longer possible on Earth without heavy water or graphite.
The Oklo uranium ore deposits are the only known sites in which natural nuclear reactors existed. Other rich uranium ore bodies would also have had sufficient uranium to support nuclear reactions at that time, but the combination of uranium, water and physical conditions needed to support the chain reaction was unique, as far as is currently known, to the Oklo ore bodies. It is also possible, that other natural nuclear fission reactors were once operating but have since been geologically disturbed so much as to be unrecognizable, possibly even "diluting" the uranium so far that the isotope ratio would no longer serve as a "fingerprint". Only a small part of the continental crust and no part of the oceanic crust reaches the age of the deposits at Oklo or an age during which isotope ratios of natural uranium would have allowed a self sustaining chain reaction with water as a moderator.
Another factor which probably contributed to the start of the Oklo natural nuclear reactor at 2 billion years, rather than earlier, was the increasing oxygen content in the Earth's atmosphere. Uranium is naturally present in the rocks of the earth, and the abundance of fissile 235
U
was at least 3% or higher at all times prior to reactor startup. Uranium is soluble in water only in the presence of oxygen.[citation needed] Therefore, increasing oxygen levels during the aging of the Earth may have allowed uranium to be dissolved and transported with groundwater to places where a high enough concentration could accumulate to form rich uranium ore bodies. Without the new aerobic environment available on Earth at the time, these concentrations probably could not have taken place.
It is estimated that nuclear reactions in the uranium in centimeter- to meter-sized veins consumed about five tons of 235
U
and elevated temperatures to a few hundred degrees Celsius. Most of the non-volatile fission products and actinides have only moved centimeters in the veins during the last 2 billion years. Studies have suggested this as a useful natural analogue for nuclear waste disposal. The overall mass defect from the fission of five tons of 235
U is about 4.6 kilograms (10 lb). Over its lifetime the reactor produced roughly 100 megatonnes of TNT (420 PJ) in thermal energy, including neutrinos. If one ignores fission of plutonium (which makes up roughly a third of fission events over the course of normal burnup in modern human-made light water reactors), then fission product yields amount to roughly 129 kilograms (284 lb) of technetium-99 (since decayed to ruthenium-99), 108 kilograms (238 lb) of zirconium-93 (since decayed to niobium-93), 198 kilograms (437 lb) of caesium-135 (since decayed to barium-135, but the real value is probably lower as its parent nuclide, xenon-135, is a strong neutron poison and will have absorbed neutrons before decaying to 135
Cs
in some cases), 28 kilograms (62 lb) of palladium-107 (since decayed to silver), 86 kilograms (190 lb) of strontium-90 (long since decayed to zirconium), and 185 kilograms (408 lb) of caesium-137 (long since decayed to barium).
Relation to the atomic fine-structure constant
The natural reactor of Oklo has been used to check if the atomic fine-structure constant α might have changed over the past 2 billion years. That is because α influences the rate of various nuclear reactions. For example, 149
Sm
captures a neutron to become 150
Sm
, and since the rate of neutron capture depends on the value of α, the ratio of the two samarium isotopes in samples from Oklo can be used to calculate the value of α from 2 billion years ago.
Several studies have analysed the relative concentrations of radioactive isotopes left behind at Oklo, and most have concluded that nuclear reactions then were much the same as they are today, which implies α was the same too.
See also
References
- The natural nuclear reactor at Oklo: A comparison with modern nuclear reactors, Radiation Information Network, April 2005
- Oklo Fossil Reactors
- NASA Astronomy Picture of the Day: NASA, Oklo, Fossile Reactor, Zone 15 (16 October 2002)
- הכור הגרעיני של הטבע (in Hebrew language)
This article uses material from the Wikipedia English article Natural nuclear fission reactor, which is released under the Creative Commons Attribution-ShareAlike 3.0 license ("CC BY-SA 3.0"); additional terms may apply (view authors). Content is available under CC BY-SA 4.0 unless otherwise noted. Images, videos and audio are available under their respective licenses.
®Wikipedia is a registered trademark of the Wiki Foundation, Inc. Wiki English (DUHOCTRUNGQUOC.VN) is an independent company and has no affiliation with Wiki Foundation.